This section goes into more detail on some of the main zones (differentiated by the main form of energy transfer taking place), between the core and the Sun's outer surface; describing the main reactions, occurring as energy slowly travels outwards, before finally being released into outer-space.
The Radiation Zone
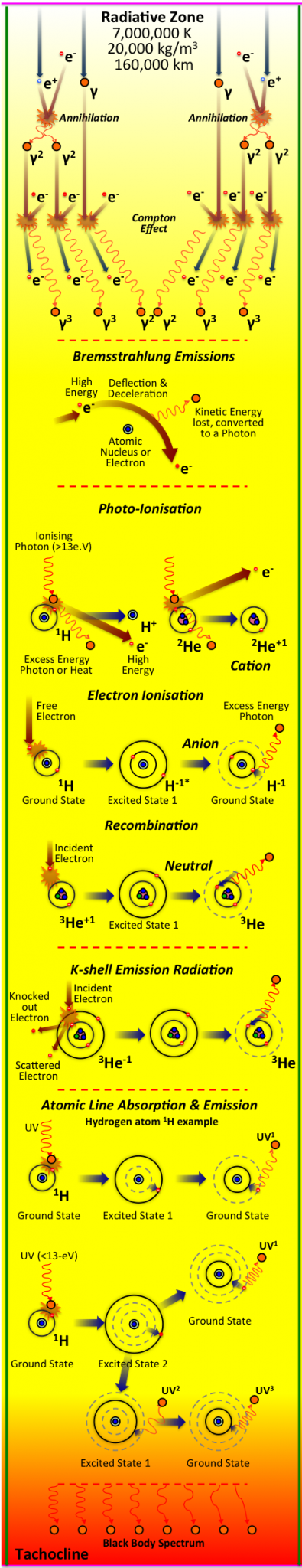
Within the Radiation Zone, the Positrons quickly encounter high attraction collisions with the many free electrons (it’s antiparticle with a negative charge), annihilating both, and giving off two or more Gamma Ray Photons in the process. Unlike the Positrons, however, all the expelled Gamma Photons are not destroyed, instead their energy is radiated (transferred) through the absorption, emission and scattering of photons in the Radiation Zone Plasma. There are four principle radiation processes in which the photons radiate across this zone, and the dominance depends mainly on temperature. In order of dominance from high to low temperature: Compton Scattering, Bremsstrahlung Emission and Absorption, Photo-ionisation and Recombination, and Atomic Line Emission and Absorption. The photons expelled from the Core, or those created through annihilation of the Positron, will soon impact with free electrons (an electron that is not bound to an atom). In a process known as Compton Scattering, the photon will scatter, while exchanging some of its energy and momentum with the free electron. This dominant process will repeat again and again, and whilst the photon is not destroyed, it is slowed, and some of its energy is lost, therefore increasing the wavelength slightly each time, and keeping the photons in thermal equilibrium with the free electrons. The next process is less to do with the expelled gamma rays, but itself creates a photon, and this is known as Bremsstahlung Emissions. Here, a high energy electron is deflected from its existing course, through the attraction or influence of another electron or atomic nucleus. During the deflection the electron looses velocity and kinetic energy, and this lost energy is released as a Photon. Atoms that contain an equal number of protons and electrons are electrically neutral; if, however, there are fewer electrons than protons, it will have a positive charge (known as a cation), or it will have a negative charge if there are more electrons than protons (known as a anion). A positively or negatively charged atom is known as an ion, and the process in which an atom (or molecule) becomes an ion is called ionisation. Within a specific range of temperatures within the Radiative Zone, another process dominates, and that is Photo-ionisation. Here, a Photon collides with an atomic electron; If the photon energy is high enough, the electron-nucleus bond will be overcome, releasing the electron free from the atom. The resulting atom is now a positively charged ion (as it has more protons than electrons) or cation. An electron can also be ‘kicked’ out of its orbit around a Nucleus by a high energy electron which can take the place of the existing electron - known as K-shell Emission Radiation (which only occur an estimated 0.2% of the time). Anions can also be created when a Free Electron collides with the shell of the atom. In this example, the electron has high energy and initially orbits at a higher energy level. But all electrons want to go to a ground state, and as there is a space available in the lower orbit, the electron will fall in to it; releasing the excess energy as a photon. This is exactly the same as the countering process to Photo-ionisation, known as Recombination; except the initial atom, prior to impact with the electron, is a Cation (and only missing one electron). So after capturing the incident electron, the atom has the same number of electrons to protons, so it becomes an eclectically neutral atom again. At lower temperatures, most of the electrons are bound to atoms, and the energy of the photons is much lower. In this situation Atomic Line Absorption and Emission is the dominant process. Here the photon energy is not sufficient to break the electron-nucleus bond, so the atomic electron absorbs the energy and rises to a higher level of orbit, but remains part of the atom. However, this may only last for a very short amount of time, as all orbiting electrons tend towards the lower energy state, and so the atomic electron quickly releases the photon or photons, and the electron returns to it’s original orbiting ground state. If the atom in the exited state impacts with another atom then this energy can be exchanged to the incident atom. Therefore, through these different radiation processes photons are scattered, or absorbed and then emitted, in random directions across the Radiation Zone (but more often outwards, towards lower temperatures and lower pressures). This journey outwards, across the Radiation Zone is known as the Random Walk; a journey which is estimated to take between 10,000 and 10,000,000 years! At the end of this journey, due to all the interactions, at the top of the Radiation Zone, the electromagnetic radiation is in thermal equilibrium with the electrons and ions, and so there is a black-body spectrum created.
Magnetic Dynamo
The Radiative Zone together with the Core rotates faster than the surrounding Zones (this is possible as the Sun is made up of plasma & gas, and is not a solid). Scientists believe this speed differential creates a very large shear, at the area of contact between these two layers, generating a huge Magnetic Dynamo – the dipolar magnetic field for the Sun (which flips its' North and South on a 11 year cycle). This area of immense shear is known as the Tachocline.
Convection Zone
After the Tachocline transition zone is the Convective Zone. Here, the visible light Photons' energy is sufficiently low, so that they can be absorbed by the solar plasma in the subsequent Convective Zone. They are not directly released, which makes the solar plasma atoms and ions (known as Granules) extremely hot. This heat makes the Granules rise to the surface – to the Photosphere – where they release their excess energy (again as Photons) creating a bubbling visible effect on the surface of the Photosphere, known as Granulation. As the energy is released, the Granules cool and then subsequently sink back down to the base of the Convection Zone, to repeat the process again. This process has been compared to a giant lava lamp.
Photosphere
As described below, the Sun has other layers above the Photosphere, however, it can be classed as the outer shell, as it is the first (lowest/deepest) surface of the Sun that is perceived to emit light. Below this layer, the plasma is opaque.
Atmosphere: Chromosphere, Transition Region, Corona, and Heliosphere
The atmosphere is composed of four distinct parts. The first, the Chromosphere, which is the 'coloured flash' that is visible at the start and end of a total solar eclipse. It is usually only visible during an eclipse, as it is not very dense, and is out-shone by the photosphere below. Many complex and dynamic phenomena, such as coronal mass ejections and coronal loops, discussed below, can be observed in the Chromosphere.
The second part of the Sun's Atmosphere, is the Solar Transition Zone. It is visible with ultraviolet sensitive telescopes, and marks the differences between the Chromosphere below and the Corona above such as: most of helium is not fully ionised below, but is above; and gas pressure and fluid dynamics seem to dominate below, however magnetic forces seem to dominate above.
The Corona is the next part of the Sun's Atmosphere. Unlike all the other layers of the Sun that get cooler the further they are from the core, the Corona actually gets much, much hotter than the visible surface of the Sun, although it is still not clear why. During quiet periods, the Corona is mainly gathered around the Sun's equator, however, during active periods, it spreads across different parts of the Sun's surface (particularly near sunspots).
And it is here that Solar Winds are formed, as superheated protons and electrons (‘smashed’ atom plasma) collect and leak out of the corona, at a rate of 7 billion tonnes per/hour, travelling at supersonic speeds.
Finally, the Heliosphere, is the outermost atmosphere of the Sun, which includes the solar wind plasma. It is described as an immense magnetic bubble-like region, which extends beyond Pluto, with its outer boundary, roughly defining the edge of the heliosphere and the interstellar gas outside the solar system.
Solar Activity: Coronal Mass Ejections, Coronal Loops, and Prominences
As well as a differing rotation speed between the Radiative Zone and the Convection Zone, the Sun also rotates at a different speed at the Equator than at the two Poles (due to it plasma like properties). This helps maintain the Magnetic Dynamo, and is also believed to twist the magnetic fields together, which over time, eventually erupt from within the Tachocline and through the Photosphere. These escaping magnetic fields form loops filled with plasma, known as Coronal Loops, and are thought to generate the Sun's intense Sunspots and Prominences.
The Sunspots appear dark on the Sun’s surface, as they are cooler than the surrounding plasma within the Photosphere (the high magnetic energy of the Coronal Loop inhibits convection), and also due to their large size (as large as 80,000km) they are visible from Earth. Prominences project out cool plasma into the Sun’s very hot exterior atmosphere – the Corona. Here they can break up and eject Gamma Rays, X-rays and Ultraviolet Rays out into space.
The Coronal Loops can also become twisted and finally break (known as Solar Flares) as they spontaneously reconfigure themselves into simpler forms, giving off energy that can also eject Gamma, X, and UV Rays into space. Of those very hot photons that were projected towards Earth, when they arrive, they can cause severe disruptions of the Earth’s upper atmosphere, creating such things as the Aurora Borealis (Northern Lights) and Geomagnetic Storms.
Coronal Mass Ejections (CME's) often follow from Solar Flares, and are usually large releases of plasma and magnetic fields from the Corona.Whilst Solar Flares are very fast, CME's are relatively slow - but have powerful effects on Earth's magnetosphere.